What Makes a Good Buffer Solution
Want this question answered. A buffer solution is one in which the pH of the solution is resistant to small additions of either a strong acid or strong base.

Tips For Iding If A Solution Is A Buffer Concept Chemistry Video By Brightstorm
Buffers can be made by adding a strong acid or base to a weak acid or base.
. Figure 622 shows the effect on the pH of an acetic acid-acetate ion buffer as the. A buffer solution is an aqueous mixture of a weak acid and its conjugate base. 10 What is the main function of a buffer solution.
17 What is buffer solution and types of buffer solution. A good buffer mixture should have about equal concentrations of both of its components. Many natural systems rely on buffering to maintain pH balance.
What Is a Buffer. 16 What determines buffer capacity. You need weak components for the buffer to work.
A buffer should not readily pass through cell membranesthis also reduces the disproportionate. The conjugate acid is created by accepting adding a proton H donated by the conjugate base. A H2SO4 is a strong acid so cannot form a buffer D is a weak acid but NaCl is not the salt of that weak acid C is perfect D is ok as the NaOH will half neutralised the acid leaving a solution containing the weak acid and the salt of that weak acid sodium acetate.
Should have the high chemical stability Low cell membrane permeability Low metal chelating capacity Should have consistent acid-base dissociation constant Low absorption spectra in UV and visible regions. There are two key terms associated with buffers. 11 How are buffer solutions made.
A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or weak base and its conjugate acid. Have a look at. Ad Buffer Solutions Products in Stock at RS.
What makes a good buffer in solution. 12 What makes a good buffer. 15 How a buffer solution maintains its pH.
Be notified when an answer is posted. For example a mixture of ammonium chloride and ammonium hydroxide acts as a buffer solution with a pH of about 925. 9 Which of the following will make a buffer solution.
A solution containing a weak base and its salt with strong acid is the basic buffer solution. What are types of buffer solutions. Buffers usually consist of a weak acid and its conjugate base in relatively equal and large quantities.
14 Which definition describes a buffer. Good buffers have a high solubility in water since most biological systems naturally use water as their solvent. If you add an acid or a base to a buffered solution its pH will not change significantly.
A buffer solution has generally lost its usefulness when the amount of one component of the buffer pair is less than about 10 of the other. Calculations are based on the equation for the ionization of the weak acid in water forming the hydronium ion and the conjugate base of the acid. This prevents the buffer from accumulating in cell membranes vesicles and other nonpolar compartments in biological systems.
When a normal quantity of strong acid or base is introduced to it the pH hardly changes. Buffer solutions are widely used in chemical applications to keep pH at a constant value. Similarly adding water to a.
There are two useful rules of thumb for selecting buffer mixtures. A solution containing a weak acid and its salts with strong base is called an acidic buffer solution. Also what makes a good buffer solution.
Therefore it is very important to be able to identify acid and conjugate base pairs. A buffer is an aqueous solution that has a highly stable pH. H NH3 NH4.
Acidic buffer are solution of a mixture of weak acid and salt of its. A buffer is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffering agent is a weak acid or weak base that helps maintain the pH of an aqueous solution after adding another acid or base.
Ad Buffer Solutions Products in Stock at RS. Wide Range of Buffer Solutions Online at RS Components. 13 What is a buffer in Chem.
Buffers should be very soluble in water and minimally so in nonpolar solvents fats oils and organic solvents. Also the solubility level of Good buffers in organic solvents such as fats and oils is low. Wide Range of Buffer Solutions Online at RS Components.
What makes a good buffer solution. If a strong acid such as HCl is added to NH 3 a weak base NH 4 the conjugate acid of NH 3 is produced. The bicarbonate buffering mechanism for example is used.

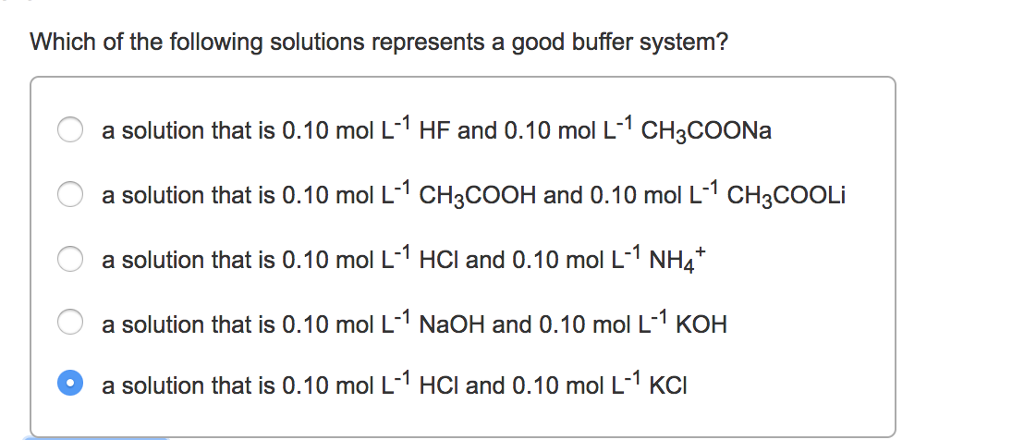
Solved Which Of The Following Solutions Represents A Good Chegg Com
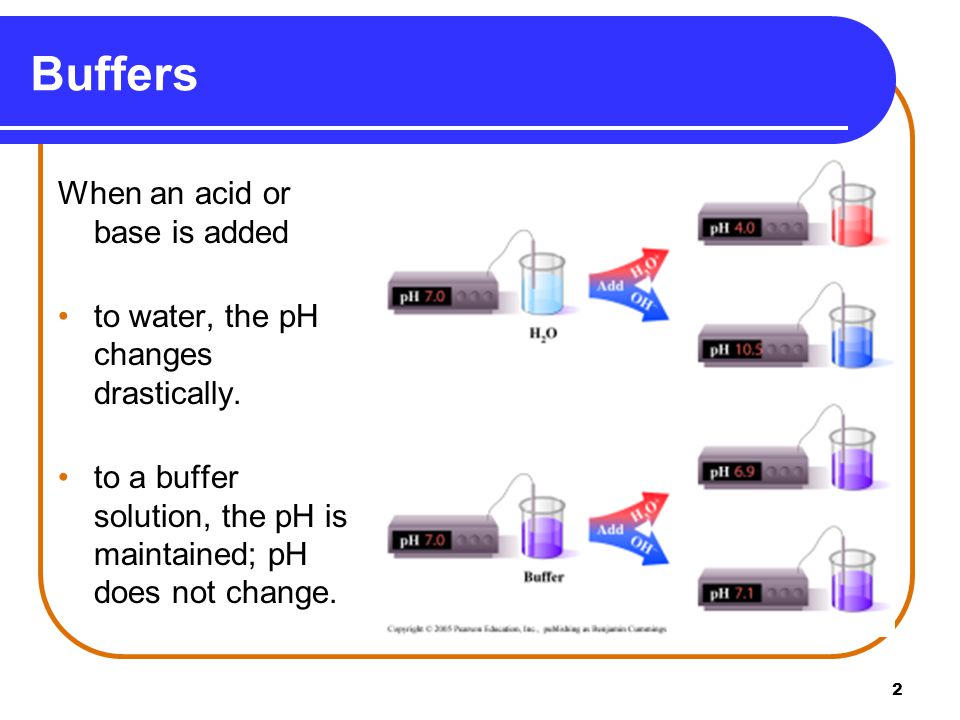
Buffer Buffering Capacity Properties Of Good Buffer And Role Of Buffer In Vitro And In Vivo Online Biology Notes

Comments
Post a Comment